

Energy Changes (such as heat/light energy is involved).It is important for students to know the four main differences between a mixture and a compound, in terms of: The components of a mixture can be separated by physical separation techniques such as filtration, simple distillation and sublimation, etc.Īn example of a widely used mixture is alloys which is defined as a mixture of a metal with other element(s) (can be metals or non-metals). water (H 2O) and carbon dioxide (CO 2)Ĭ) Element and compound e.g. helium (He) and oxygen (O 2)ī) Two compounds e.g. they can be present in any ratio.Ī) Two elements e.g. The composition of a mixture are not fixed i.e. Mixtures can be made up of elements or compounds. This is different from elements and compounds. Mixtures do not have a chemical formula to represent it. Reduce the “subscript” to the lowest termĪ mixture is made up of two or more substances that are not chemically combined. Oxygen atom is usually written at the end No of atoms of elements is written as subscripts Symbol of Metallic Element is written 1st Tips on writing chemical formula of compound: Rules ratio of the different atoms present in the compound.The chemical formula of a compound can be determined by putting the formula of the elements that made up the compound. CompoundĬompounds can also be represented by chemical formulae. For example, carbon dioxide is a compound made up of molecules while sodium chloride is a compound made up of positive sodium ions and negative chloride ions.Ĭompounds have a chemical name which indicate the types of elements present. B) CompoundsĪ pure substance that contains two or more elements chemically combined together in a fixed ratio.Ī compound can be decomposed by chemical processes such as thermal decomposition and electrolysis (which is the process of using electricity to break down a compound).Ī compound may be made up of molecules (covalent compound) or ions (ionic compound). Common example will be ozone (O 3) which is triatomic. Polyatomic molecules are those that contain three or more atoms. Elements which exists as diatomic elements are hydrogen (H 2), oxygen (O 2), nitrogen (N 2), chlorine (Cl 2), etc.

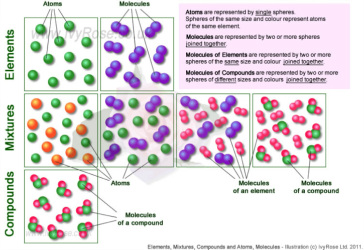
they exists as individual atoms.Ī molecule is a group of two or more atoms that are chemically combined together.ĭiatomic molecules refers those that are formed by the combination of two atoms. Group 0 Noble Gases such as helium, neon, argon, kryton and xenon are known as monoatomic elements i.e. Si and GeĮlements can exist as atoms or molecules.Īn atom is the smallest particle of an element that has the chemical properties of an element.Įach element contains only one type of atom. Metalloids have properties of both metals and non-metals.
Element definition and example full#
You should refer to the Periodic Table for the full list of elements.Įlements can be classified in different ways:
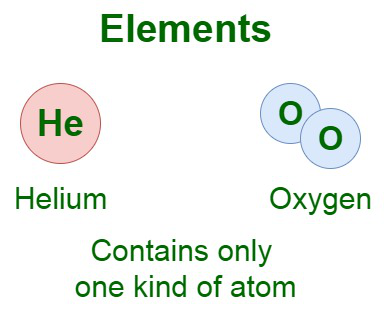
Very often, you can guess the chemical symbol from the first two letters of the element’s chemical name. The names and chemical symbols of some common elements are listed below: It is a pure substance that cannot be split into two or more simpler substances by chemical means.įor example, carbon, hydrogen and oxygen are known as elements since they cannot be broken down further into simpler substances.Įlements are represented by chemical names & chemical symbols. You need to know their definitions and properties very well in order to do so. So, how do we tell if a substance is an element, a compound or a mixture? Matters can also be classified as Elements, Compounds & Mixtures. Matters has been classified as 3 states of matter, namely Solid, Liquid & Gas.


 0 kommentar(er)
0 kommentar(er)
